Publication of Peter Strasser has been highlighted in JACS
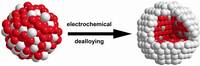
Chemical dealloying, a process that selectively leaches one metal out of an alloy — a two-metal mixture — has recently emerged as way to synthesize nanosized particles composed of a core rich in the less noble metal, like cobalt or copper, surrounded by a shell of the more noble one, like platinum.
This type of core–shell nanoparticle with a platinum shell can act as a catalyst in fuel cell reactions, in which chemical energy from a fuel is turned into electricity. Dealloyed nanoparticles may have catalytic activity, resulting in more efficient fuel cells, but the atomic process during the dealloying is not fully understood.
Mehtap Oezaslan, Marc Heggen, and Peter Strasser determined the structure of dealloyed core–shell catalysts of various sizes using Pt–Co and Pt–Cu alloy nanoparticle precursors. The researchers leached Co or Cu from the particle surfaces, leaving behind a pure Pt shell, and they found that particle size had a significant impact on morphology and composition.
The smallest particles, less than 5 nm in diameter, formed spheres of a platinum-poor alloy core surrounded by a platinum shell. However, as size increased to up to 10 nm, the nanoparticles contained multiple metal cores.
The authors suggest that these findings close the gap in our understanding between the morphology of highly active Pt nanoparticle catalysts for fuel cells and the corrosion of macroscale metal alloys in industrial settings.
Adapted from: Christen Brownlee; JACS Spotlights.
The original publication by Oezaslan et al. is titled: Size-Dependent Morphology of Dealloyed Bimetallic Catalysts: Linking the Nano to the Macro Scale.